BES2019 BES 2019 Effect of nationwide reimbursement of sensor-augmented pump therapy in a paediatric type 1 diabetes population on HBA1C, hypoglycaemia and quality of life: the rescue-paediatrics study (1 abstracts)
Effect of nationwide reimbursement of sensor-augmented pump therapy in a paediatric type 1 diabetes population on HBA1C, hypoglycaemia and quality of life: The rescue-paediatrics study
F De Ridder *,1,2,3 , S Charleer *,4,5 , S Jacobs 1,2,3 , K Casteels 6 , S Van Aken 7 , J Vanbesien 8 , I Gies 8 , G Massa 9 , P Lysy 10 , K Logghe 11 , M-C Lebrethon 12 , S Depoorter 13 , K Ledeganck 3 , P Gillard 2,4,14 , C De Block †,2,3 , M den Brinker †,1,3 & on behalf of the RESCUE trialists
1Department of Paediatric Endocrinology, Antwerp University Hospital, Edegem, Belgium; 2Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Edegem, Belgium; 3University of Antwerp, Edegem, Belgium; 4Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium; 5Fund for Scientific Research (FWO), SB PhD fellow, Brussels, Belgium; 6Department of Paediatric Endocrinology, University Hospitals Leuven, Leuven, Belgium; 7Department of Paediatric Endocrinology, University Hospital Ghent, Gent, Belgium; 8Department of Paediatric Endocrinology, University Hospital Brussels, Jette, Belgium; 9Department of Paediatric Endocrinology, Jessa Hospital, Hasselt, Belgium; 10Department of Paediatric Endocrinology, Saint-Luc Hospital Brussels, Woluwe-Saint-Lambert, Belgium; 11Department of Paediatric Endocrinology, General Hospital Delta, Delta, Canada; 12Department of Paediatric Endocrinology, Regional Center Hospital Liège, Brussels, Belgium; 13Department of Paediatric Endocrinology, General Hospital Sint-Jan Bruges, Brugge, Belgium; 14Fund for Scientific Research (FWO), Senior clinical investigator fellow, Brussels. *Joint First Authorship; †Joint Last Authorship; ‡RESCUE study, ClinicalTrials.gov: NCT02601729.
Background: Long-term real-life data of sensor-augmented pump therapy (SAP) in paediatric type 1 diabetes (T1D) patients are lacking.


Objectives: To assess the impact of SAP in a nationwide study of paediatric T1D patients on HbA1c, hypoglycaemia and quality of life until 24 months.
Methods: Between December 2014 and February 2017, 75 children entered the Belgian reimbursement system for SAP and were followed for 12 (n=73) and 24 months (sub analysis of the children of the University Hospital of Antwerp; n=25). Study endpoints included evolution of HbA1c, hypoglycaemia and quality of life (impact, satisfaction, worry and parents’ questionnaires).
Results: Seventy-three (97%) patients used SAP for 12 months. Baseline HbA1c (7.2±0.7%) decreased to 7.1±0.8% at 4 months (P=0.02), remained stable at 8 months (7.1%±0.7%; P=0.03) and 12 months (7.1%±0.8%; P=0.1), even after 24 months (7.1±0.9%; P=0.4). Patients with a baseline HbA1c <7.5% (n=46), had a mean HbA1c of 6.8±0.5% and it did not change after 4, 8, 12 and 24 months (P=NS). Subjects with a baseline HbA1c ≥7.5% (n=26, mean HbA1c 8.0±0.4%) showed an improvement of 0.4% at 4 months (7.6±0.7%; P=0.002), an improvement of 0.5% at 8 months (7.5±0.6%; P=0.001), with a stabilisation at 12 months (7.6±0.7%; P=0.03). No improvement was seen after 24 months in the remaining seven children with a poor metabolic control (8.0±1.0%; P=0.9). Time in hypoglycaemia (50–70 mg/dl) decreased from 5.8±5.1% at baseline to 4.8±3.2% at 12 months (P=0.07) and to 3.5±4.0% at 24 months (n=20; P=0.009). Time in severe hypoglycaemia (<50 mg/dl) did not change over time (1.0±1.6%). Overall, quality of life did not change in our patients while using SAP.
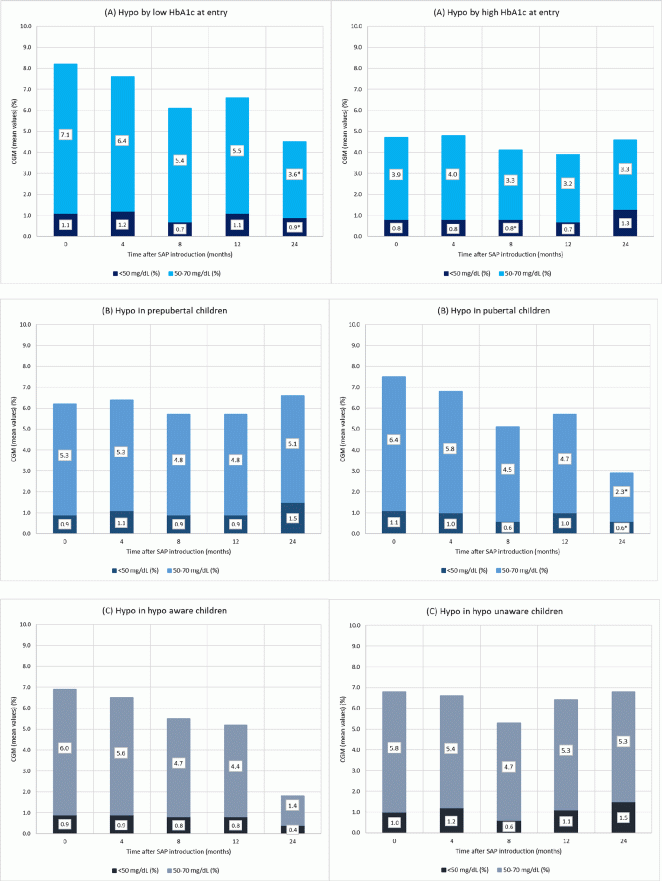
Conclusions: Reimbursement of SAP in paediatric T1D patients improved HbA1c, especially in patients with a poor metabolic control, and decreased time in hypoglycaemia without affecting overall quality of life.






